TABLE OF CONTENTS
Carbon dioxide transport in respiration
The CO2 produced during metabolism is a waste product and has been eliminated. The flow of CO2 is effected under a continuous pressure gradient.

The transport of CO2 is effected in the following ways as physically dissolved CO2 and transport in chemical combination.
1. Physically dissolved CO2
Compared with O2, CO2 is 24 times more soluble in blood plasma. Only about 5 – 7 % of total CO2 carried by blood is in a simple physical solution. The factors that determine the transport are the partial pressure of CO2 (Henry’s law) and temperature. Both plasma and cells can transport CO2 in a physically dissolved state. (The solubility factor per litre for CO2 at 380C is 0.03 for plasma and 0.025 for cells).
2. Transport in chemical combination
By far the greater proportion of CO2 is transported in blood in chemical combination as HCO3. Majority of the CO2 diffusing into the blood combines with water forms carbonic acid, which ten rapidly dissociates into HCO3ions and H+. This reversible reaction is kept moving to the right because the H+ is buffered by plasma proteins / Hb.
Formation of HCO3 in plasma
The Chief buffers of plasma that provides for buffering action by combining with H+ is protein system (Proteinate / Protein).
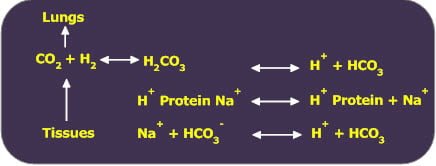
The PCO2 in tissues is higher than in arterial blood causes diffusion of CO2 into blood and forces the preceding reversible reaction to the right causing an increase in HCO3. This increase in HCO3 represents transport due to plasma proteins.
When venous blood reaches the lungs, CO2 diffuses into alveolar air and is exhaled out. This causes the preceding reaction to shift to left.
The pH of arterial plasma decreases about 0.025 units in becoming venous plasma.
The transport of CO2 as HCO3– by plasma proteins represents about 4% of total CO2 transport.
Formation of HCO3 by RBCs (Isohydric Transport of CO2)
The CO2 from tissues enter plasma then into the RBCs. H2CO3 formation by the reaction of CO2 with water is too slow to less of importance in plasma but inside the RBCs, the enzyme carbonic anhydrase catalyses the reaction between CO2 and H2O accelerating the rate of about 5,000 fold.
H2CO3 rapidly and spontaneously dissociates into H+ and HCO3. Since an increase in H+ concentration is severely detrimental to an organism, a base buffer must be available to remove the H+ ions. The Hb provides the buffering, driving the reaction to the right.
In the tissue Oxy-Hb delivers O2 and becomes deoxygenated Hb. The deoxygenated Hb is a weaker acid than Oxy-Hb and function as a better buffer readily combines with H+ and facilitates the formation of HCO3 form CO2.
Hb is a major buffer in blood that removes free H+ from blood and an equal quantity of HCO3 is left dissolved in fluid. The HCO3 diffuse out of RBC into the plasma due to concentration gradient by exchanging choloride ions from the plasma across the RBC membrane referred as chloride shift or Hamburger shift.
The conversion of CO2 via H2CO3 to HCO3 ion in erythrocytes accounts for 70% for CO2transport.
In the lungs, high PO2 favours diffusion of O2 into the red cells and oxygenates Hb. On oxygenation HHb becomes Oxy Hb and releases H+ ions. This H+ ion combine with HCO3 and drives the reaction to the left.
Transport as carbamino hemoglobin
Haemoglobin contains terminal amino group (NH) reversibly react with CO2 to from an unstable carbamino compound.
About 20% CO2 is transported as carbamino haemoglobin (Though plasma proteins can also form carbamino compounds, this reaction in plasma is negligible because free NH group is relatively less in plasma proteins).